Makindo Medical Notes"One small step for man, one large step for Makindo" |
|
|---|---|
| Download all this content in the Apps now Android App and Apple iPhone/Pad App | |
| MEDICAL DISCLAIMER: The contents are under continuing development and improvements and despite all efforts may contain errors of omission or fact. This is not to be used for the assessment, diagnosis, or management of patients. It should not be regarded as medical advice by healthcare workers or laypeople. It is for educational purposes only. Please adhere to your local protocols. Use the BNF for drug information. If you are unwell please seek urgent healthcare advice. If you do not accept this then please do not use the website. Makindo Ltd. |
Myoglobin
-
| About | Anaesthetics and Critical Care | Anatomy | Biochemistry | Cardiology | Clinical Cases | CompSci | Crib | Dermatology | Differentials | Drugs | ENT | Electrocardiogram | Embryology | Emergency Medicine | Endocrinology | Ethics | Foundation Doctors | Gastroenterology | General Information | General Practice | Genetics | Geriatric Medicine | Guidelines | Haematology | Hepatology | Immunology | Infectious Diseases | Infographic | Investigations | Lists | Microbiology | Miscellaneous | Nephrology | Neuroanatomy | Neurology | Nutrition | OSCE | Obstetrics Gynaecology | Oncology | Ophthalmology | Oral Medicine and Dentistry | Paediatrics | Palliative | Pathology | Pharmacology | Physiology | Procedures | Psychiatry | Radiology | Respiratory | Resuscitation | Rheumatology | Statistics and Research | Stroke | Surgery | Toxicology | Trauma and Orthopaedics | Twitter | Urology
Related Subjects: |Heme |Globins |Myoglobin |Iodine |Cell Adhesion Molecules |B Lymphocytes
Myoglobin is a globular protein found primarily in muscle tissue, where it serves as an oxygen-storage unit, providing oxygen to the working muscles. It is structurally similar to haemoglobin, the oxygen-carrying protein in red blood cells, but myoglobin has a higher affinity for oxygen and functions in muscle cells to facilitate oxygen diffusion.
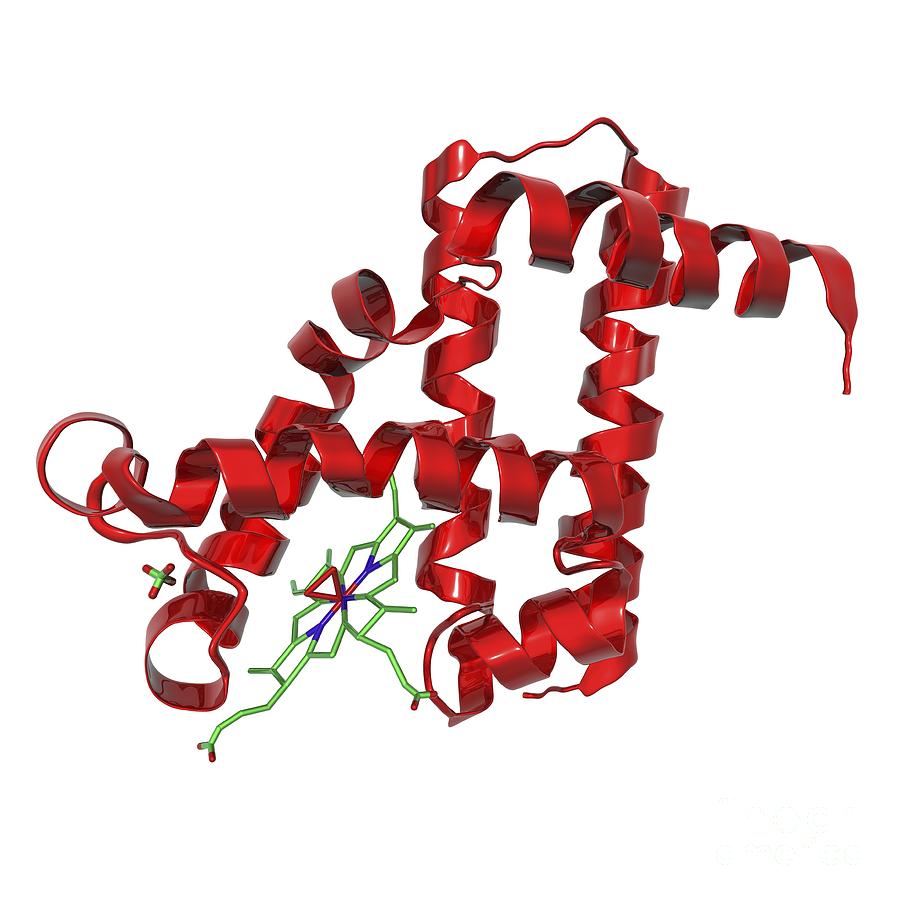
Structure of Myoglobin
- Myoglobin is a monomeric protein consisting of a single polypeptide chain.
- Composed of 153 amino acids.
- Contains a heme prosthetic group, which is responsible for binding oxygen.
- The heme group consists of an iron ion (Fe2+) coordinated within a porphyrin ring.
- The protein has a compact, globular structure, primarily composed of alpha-helices (eight helices labeled A to H).
Function of Myoglobin
- Oxygen Storage :
- Myoglobin stores oxygen in muscle cells, particularly in skeletal and cardiac muscles, where it provides a readily available supply during periods of high metabolic demand.
- Oxygen Transport :
- Facilitates the diffusion of oxygen from the blood to the mitochondria of muscle cells.
- Ensures a continuous supply of oxygen for aerobic respiration and ATP production in muscle tissues.
Oxygen Binding and Release
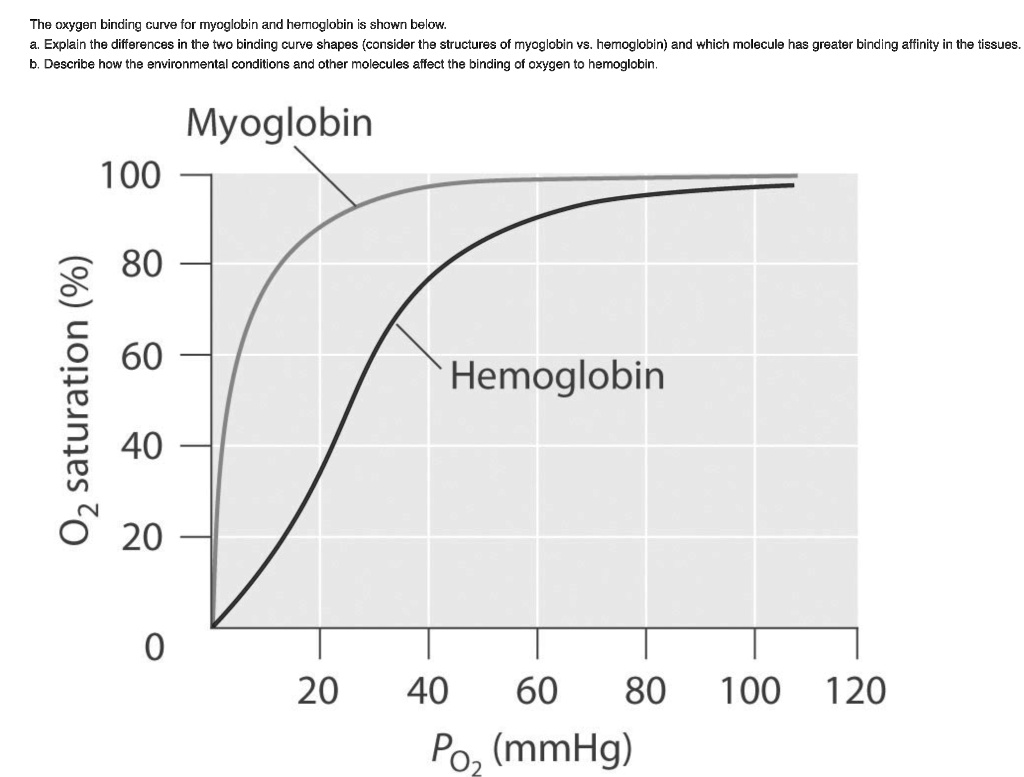
- Myoglobin has a higher affinity for oxygen than haemoglobin.
- Oxygen binding to myoglobin is described by a hyperbolic curve, indicating a single binding site for oxygen.
- Myoglobin becomes saturated with oxygen at lower partial pressures compared to haemoglobin, allowing it to effectively capture and store oxygen in muscle tissues.
- Myoglobin releases oxygen when the partial pressure of oxygen in muscle cells decreases during intense physical activity.
- This release mechanism ensures that muscles receive an adequate supply of oxygen during periods of high demand.
Comparison with Haemoglobin
- Structure :
- Myoglobin is a monomer, while haemoglobin is a tetramer composed of two alpha and two beta subunits.
- Oxygen Affinity :
- Myoglobin has a higher affinity for oxygen and binds oxygen more tightly than haemoglobin.
- Haemoglobin exhibits cooperative binding, resulting in a sigmoidal oxygen dissociation curve, whereas myoglobin displays a hyperbolic curve.
- Function :
- Myoglobin primarily serves as an oxygen storage protein in muscle tissues.
- Haemoglobin is responsible for oxygen transport in the blood from the lungs to tissues throughout the body.
Clinical Relevance
- Myoglobinuria :
- Condition characterized by the presence of myoglobin in the urine, often due to muscle injury or rhabdomyolysis.
- Can lead to kidney damage and acute renal failure if not treated promptly.
- Myocardial Infarction :
- Myoglobin is released into the bloodstream following myocardial injury, such as during a heart attack.
- Elevated levels of myoglobin in blood tests can serve as an early marker for myocardial infarction.
Summary
Myoglobin is a crucial protein for oxygen storage and transport in muscle tissues, ensuring an adequate supply of oxygen during periods of high metabolic activity. Its structure, function, and clinical significance are essential for understanding muscle physiology and the diagnosis of related medical conditions. The higher affinity of myoglobin for oxygen compared to haemoglobin makes it uniquely suited for its role in muscle cells.