Makindo Medical Notes"One small step for man, one large step for Makindo" |
|
|---|---|
| Download all this content in the Apps now Android App and Apple iPhone/Pad App | |
| MEDICAL DISCLAIMER: The contents are under continuing development and improvements and despite all efforts may contain errors of omission or fact. This is not to be used for the assessment, diagnosis, or management of patients. It should not be regarded as medical advice by healthcare workers or laypeople. It is for educational purposes only. Please adhere to your local protocols. Use the BNF for drug information. If you are unwell please seek urgent healthcare advice. If you do not accept this then please do not use the website. Makindo Ltd. |
Barrett's oesophagus
-
| About | Anaesthetics and Critical Care | Anatomy | Biochemistry | Cardiology | Clinical Cases | CompSci | Crib | Dermatology | Differentials | Drugs | ENT | Electrocardiogram | Embryology | Emergency Medicine | Endocrinology | Ethics | Foundation Doctors | Gastroenterology | General Information | General Practice | Genetics | Geriatric Medicine | Guidelines | Haematology | Hepatology | Immunology | Infectious Diseases | Infographic | Investigations | Lists | Microbiology | Miscellaneous | Nephrology | Neuroanatomy | Neurology | Nutrition | OSCE | Obstetrics Gynaecology | Oncology | Ophthalmology | Oral Medicine and Dentistry | Paediatrics | Palliative | Pathology | Pharmacology | Physiology | Procedures | Psychiatry | Radiology | Respiratory | Resuscitation | Rheumatology | Statistics and Research | Stroke | Surgery | Toxicology | Trauma and Orthopaedics | Twitter | Urology
Related Subjects: |Achalasia |Oesophageal Carcinoma |Diffuse Oesophageal spasm |Diffuse Oesophageal Perforation - Rupture |Gastro-Oesophageal Reflux |Barrett's oesophagus
🧪 Barrett's oesophagus is a premalignant condition where the normal squamous epithelium of the distal oesophagus is replaced by specialised intestinal-type columnar epithelium due to chronic acid exposure from GORD. ⚠️ This metaplastic change increases the lifetime risk of developing oesophageal adenocarcinoma.
📌 About
- Defined as endoscopically visible columnar epithelium extending ≥1 cm above the gastro-oesophageal junction (GOJ), confirmed histologically.
- Normal pale-grey squamous lining → replaced by salmon-pink columnar mucosa.
- Diagnosis requires both endoscopic evidence and biopsy confirmation.
🧬 Aetiology
- Chronic GORD → repeated acid/bile reflux damages squamous lining.
- Healing occurs with intestinal metaplasia (columnar epithelium with goblet cells).
- Risk factors: Male sex, >45 years, Caucasian ethnicity, central obesity, smoking, and chronic reflux symptoms.
🔥 Risk of Malignant Transformation
| Risk Factor | Increased Cancer Risk? |
|---|---|
| Male sex | ↑ |
| Long-segment Barrett's (>8 cm) | ↑↑ |
| High-grade dysplasia | Very high |
| Smoking & obesity | ↑ |
| Family history of oesophageal cancer | ↑ |
🔎 Investigations
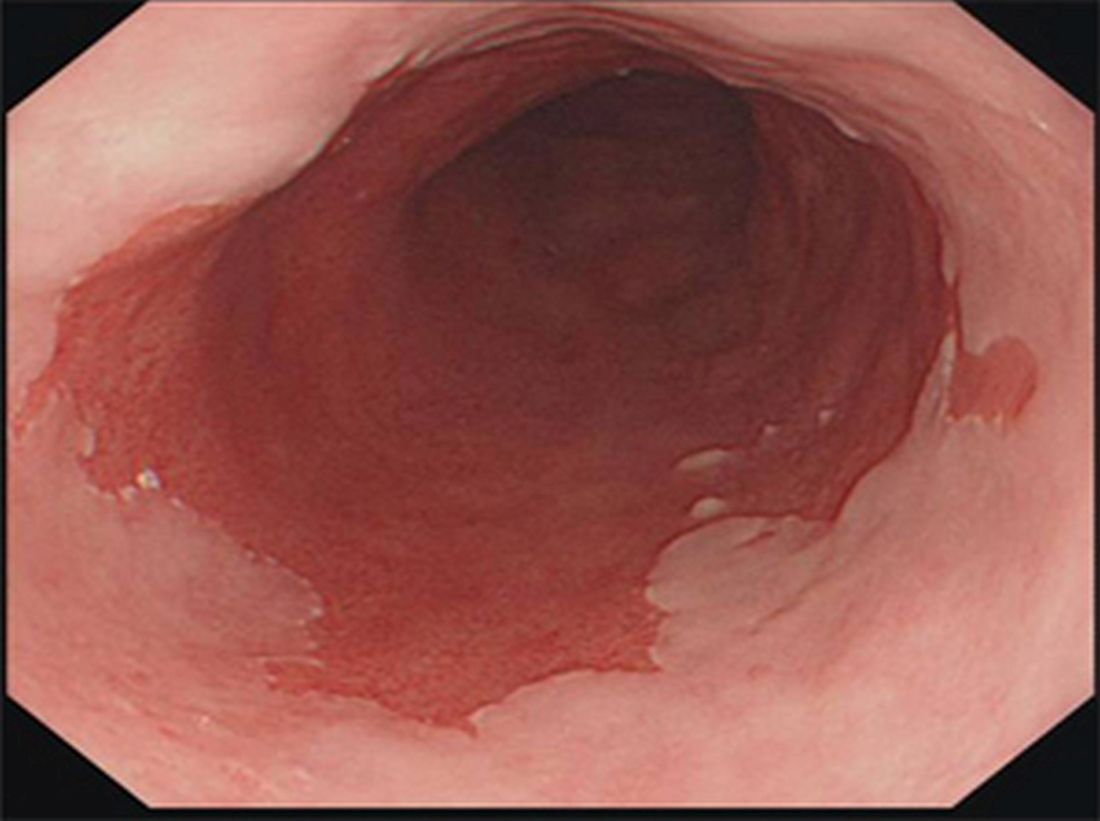
- Endoscopy: Columnar epithelium appears salmon-pink vs normal pale-grey squamous mucosa.
- Seattle protocol biopsies: Quadrantic biopsies every 2 cm (or 1 cm if dysplasia suspected).
- Histology: Assess for goblet cells and grade dysplasia:
- No dysplasia
- Low-grade dysplasia
- High-grade dysplasia
- Adjuncts: Chromoendoscopy (e.g. indigo carmine) to highlight dysplasia.
💊 Management
- Lifestyle & diet: Weight loss, avoid alcohol, caffeine, spicy foods, elevate head of bed, smoking cessation.
- Acid suppression: Long-term PPIs (high dose if symptoms persist). H2 antagonists are less effective.
- Endoscopic surveillance: Based on length and dysplasia grade (see NICE below).
- Endoscopic therapy:
- Radiofrequency ablation (RFA) → first-line for dysplasia.
- Endoscopic mucosal resection (EMR) → removes visible lesions.
- Cryotherapy → alternative for ablation.
- Surgery: Oesophagectomy for high-grade dysplasia or intramucosal carcinoma where endoscopic therapy not suitable.
📏 Screening & Surveillance (NICE)
- Long-segment (≥3 cm): Endoscopy every 2–3 years.
- Short-segment (<3 cm) with intestinal metaplasia: Endoscopy every 3–5 years.
- No intestinal metaplasia confirmed: No surveillance after 2 negative endoscopies.
- Indefinite for dysplasia: Optimise acid suppression → repeat in 6 months.
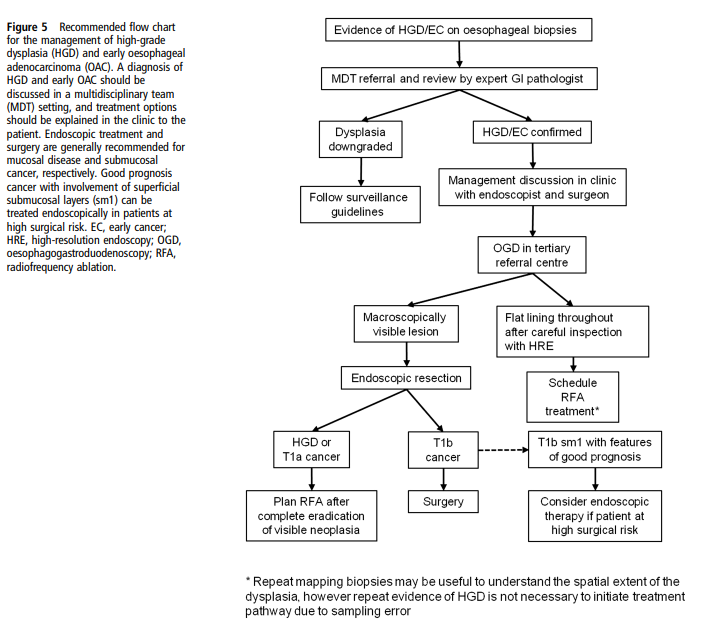
- High-grade dysplasia → endoscopic eradication therapy preferred over surveillance alone.
📚 References
- BSG Guidelines on Diagnosis & Management of Barrett’s Oesophagus
- NICE CG106: Barrett’s Oesophagus and Oesophageal Cancer
Cases — Barrett’s Oesophagus
- Case 1 (Non-dysplastic): A 44-year-old man with long-standing reflux presents with heartburn despite PPI use. Endoscopy shows a 3 cm segment of salmon-coloured mucosa above the gastro-oesophageal junction. Biopsies confirm intestinal metaplasia without dysplasia.
Management: Optimised high-dose PPI therapy, lifestyle advice (weight loss, alcohol/caffeine avoidance), and enrolment into endoscopic surveillance programme every 3–5 years. Outcome: Symptoms improve with medication. No progression on repeat endoscopy at 3 years. - Case 2 (Low-grade dysplasia): A 61-year-old man with obesity and reflux symptoms undergoes endoscopy for persistent dyspepsia. A 4 cm Barrett’s segment is seen; histology shows low-grade dysplasia. Management: Referred for endoscopic radiofrequency ablation (RFA) after PPI optimisation. Close endoscopic surveillance arranged at 6–12 month intervals. Outcome: Successful eradication of dysplastic tissue with RFA. Remains under annual endoscopic surveillance.
- Case 3 (High-grade dysplasia/early cancer): A 72-year-old man presents with progressive dysphagia and weight loss. Endoscopy reveals a nodular Barrett’s segment; biopsies show high-grade dysplasia with intramucosal carcinoma. Management: Endoscopic mucosal resection (EMR) of the lesion, followed by RFA of residual Barrett’s segment. Multidisciplinary team (MDT) review confirms no invasive cancer. Outcome: Good symptom relief, no recurrence at 1-year follow-up. Enrolled in close surveillance programme.
Teaching Commentary 🧑⚕️
Barrett’s oesophagus is the replacement of normal squamous epithelium by intestinal-type columnar epithelium due to chronic reflux. It is a precancerous condition with risk of oesophageal adenocarcinoma. Management depends on histology: • Non-dysplastic → PPI + surveillance. • Low-grade dysplasia → endoscopic therapy (RFA) ± close monitoring. • High-grade dysplasia / intramucosal carcinoma → EMR + RFA or surgery if invasive. Lifestyle modification is essential in all cases. Long-term follow-up is required, as progression risk is ~0.1–1% per year depending on dysplasia grade.