Makindo Medical Notes"One small step for man, one large step for Makindo" |
|
|---|---|
| Download all this content in the Apps now Android App and Apple iPhone/Pad App | |
| MEDICAL DISCLAIMER: The contents are under continuing development and improvements and despite all efforts may contain errors of omission or fact. This is not to be used for the assessment, diagnosis, or management of patients. It should not be regarded as medical advice by healthcare workers or laypeople. It is for educational purposes only. Please adhere to your local protocols. Use the BNF for drug information. If you are unwell please seek urgent healthcare advice. If you do not accept this then please do not use the website. Makindo Ltd. |
Haemoglobins
-
| About | Anaesthetics and Critical Care | Anatomy | Biochemistry | Cardiology | Clinical Cases | CompSci | Crib | Dermatology | Differentials | Drugs | ENT | Electrocardiogram | Embryology | Emergency Medicine | Endocrinology | Ethics | Foundation Doctors | Gastroenterology | General Information | General Practice | Genetics | Geriatric Medicine | Guidelines | Haematology | Hepatology | Immunology | Infectious Diseases | Infographic | Investigations | Lists | Microbiology | Miscellaneous | Nephrology | Neuroanatomy | Neurology | Nutrition | OSCE | Obstetrics Gynaecology | Oncology | Ophthalmology | Oral Medicine and Dentistry | Paediatrics | Palliative | Pathology | Pharmacology | Physiology | Procedures | Psychiatry | Radiology | Respiratory | Resuscitation | Rheumatology | Statistics and Research | Stroke | Surgery | Toxicology | Trauma and Orthopaedics | Twitter | Urology
Related Subjects:
|Iron deficiency Anaemia
|Haemolytic anaemia
|Macrocytic anaemia
|Megaloblastic anaemia
|Microcytic anaemia
|Myelodysplasia
|Myelofibrosis
🩸 Haemoglobin is a vital protein in red blood cells that transports oxygen from the lungs to tissues and returns carbon dioxide for exhalation. It is essential for maintaining tissue oxygenation and overall metabolic function.
💡 Key Point: Haem is essential not only for haemoglobin but also for myoglobin and cytochromes. Synthesis occurs in the bone marrow & liver.
💡 Exam Tip: “Right shift = Release” (easy way to remember).
Haemoglobin = 🩸 oxygen transporter, CO₂ remover, and pH buffer.
Its complex structure allows precise regulation. Disorders of synthesis (porphyrias, lead poisoning) or structure (sickle cell, thalassemias) underline its clinical importance.
Understanding shifts in the O₂ curve and genetic variants is high-yield for exams and practice.
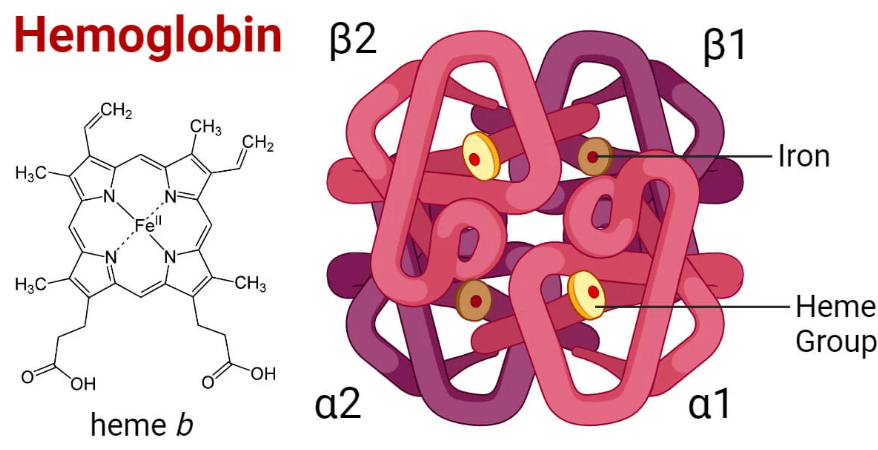
🔬 Structure of Haemoglobin
⚙️ Formation of Haem
🧪 Steps in Haem Synthesis
⚖️ Regulation
📚 Clinical Relevance of Haem Synthesis
🧬 Types of Haemoglobin
Type Composition Normal Proportion Key Features
HbA α₂β₂ ~97% Normal adult haemoglobin
HbA₂ α₂δ₂ ~2-3% Slightly higher in β-thalassemia trait
HbF α₂γ₂ <1% adults
Predominant in fetusHigher O₂ affinity → helps placental transfer
HbS Mutated β chain Pathological Sickle cell disease
HbC Mutated β chain Pathological HbC disease with haemolysis
🧭 Function of Haemoglobin
📈 Oxygen–Haemoglobin Dissociation Curve
🩸 Clinical Relevance
📝 Summary