Makindo Medical Notes"One small step for man, one large step for Makindo" |
|
|---|---|
| Download all this content in the Apps now Android App and Apple iPhone/Pad App | |
| MEDICAL DISCLAIMER: The contents are under continuing development and improvements and despite all efforts may contain errors of omission or fact. This is not to be used for the assessment, diagnosis, or management of patients. It should not be regarded as medical advice by healthcare workers or laypeople. It is for educational purposes only. Please adhere to your local protocols. Use the BNF for drug information. If you are unwell please seek urgent healthcare advice. If you do not accept this then please do not use the website. Makindo Ltd. |
Cardiac Anatomy and Physiology
-
| About | Anaesthetics and Critical Care | Anatomy | Biochemistry | Cardiology | Clinical Cases | CompSci | Crib | Dermatology | Differentials | Drugs | ENT | Electrocardiogram | Embryology | Emergency Medicine | Endocrinology | Ethics | Foundation Doctors | Gastroenterology | General Information | General Practice | Genetics | Geriatric Medicine | Guidelines | Haematology | Hepatology | Immunology | Infectious Diseases | Infographic | Investigations | Lists | Microbiology | Miscellaneous | Nephrology | Neuroanatomy | Neurology | Nutrition | OSCE | Obstetrics Gynaecology | Oncology | Ophthalmology | Oral Medicine and Dentistry | Paediatrics | Palliative | Pathology | Pharmacology | Physiology | Procedures | Psychiatry | Radiology | Respiratory | Resuscitation | Rheumatology | Statistics and Research | Stroke | Surgery | Toxicology | Trauma and Orthopaedics | Twitter | Urology
Related Subjects: | Cardiac Anatomy and Physiology | Coronary Artery Anatomy and Physiology | Cardiac Electrophysiology | Cardiac Embryology
The heart is a muscular, cone-shaped organ located in the middle mediastinum of the thoracic cavity. Encased in the fibrous pericardium, it rests between the lungs on the diaphragm. Oriented obliquely, the heart’s apex points downward and to the left—typically at the 5th intercostal space along the mid-clavicular line—while its base faces posteriorly toward the vertebral column.
📌 Exam Tip: Apex beat normally felt at 5th intercostal space, MCL. Lateral displacement suggests LVH (e.g., in hypertension or aortic stenosis).
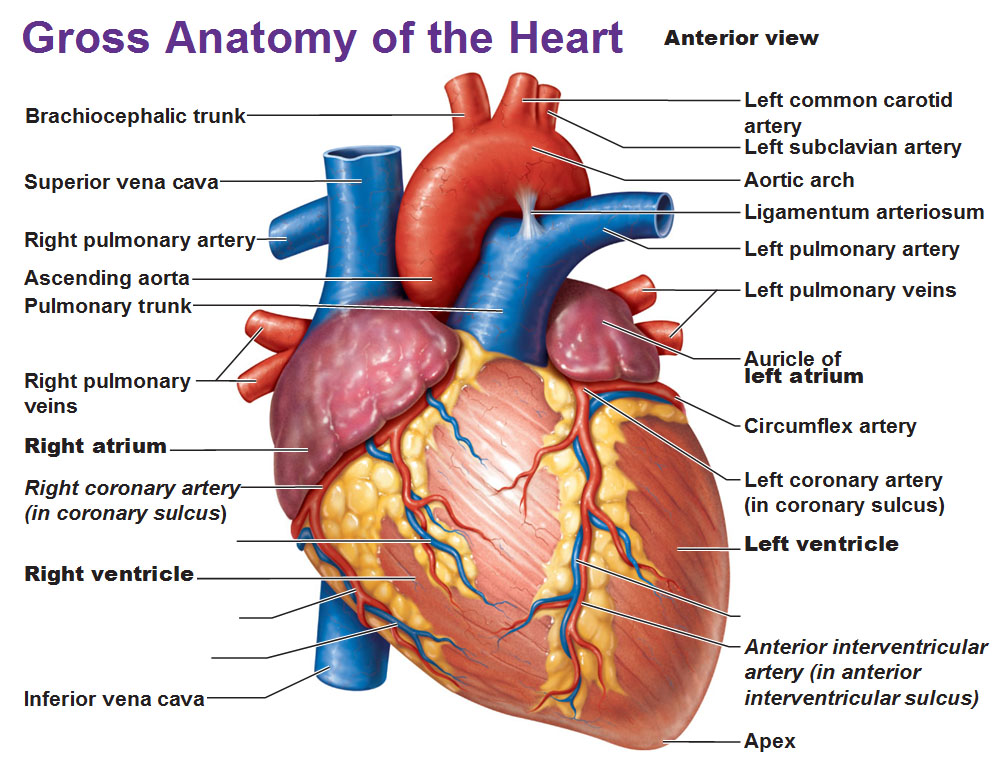
Structural Overview 🏗️
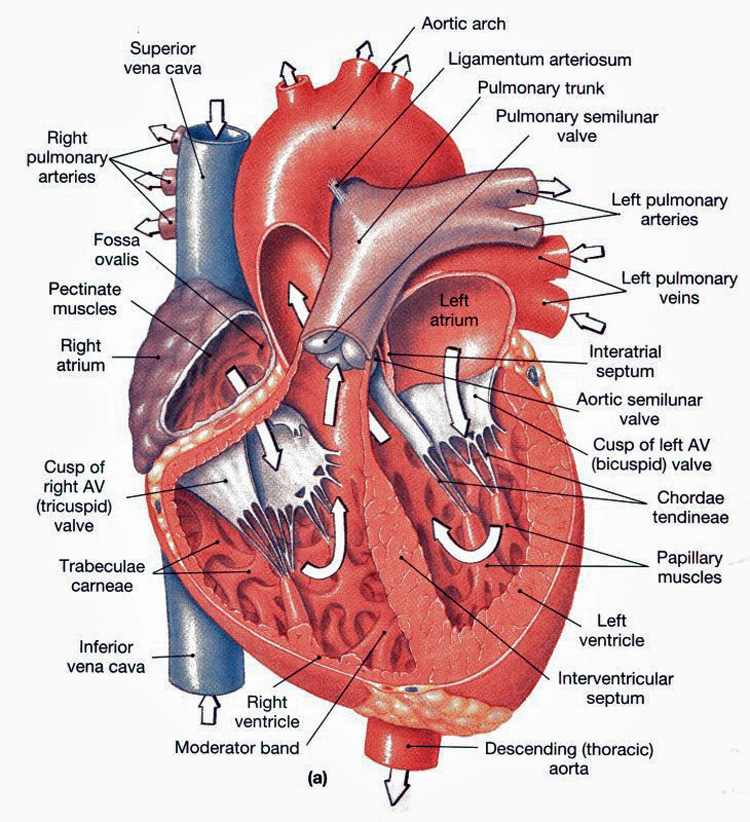
- Chambers: The heart has 4 chambers:
- 🫀 Right Atrium: Receives deoxygenated blood from SVC, IVC, coronary sinus.
- ➡️ Right Ventricle: Pumps deoxygenated blood via pulmonary valve → lungs.
- 🫁 Left Atrium: Receives oxygenated blood from pulmonary veins.
- 💪 Left Ventricle: Thickest wall, pumps oxygenated blood via aortic valve → systemic circulation.
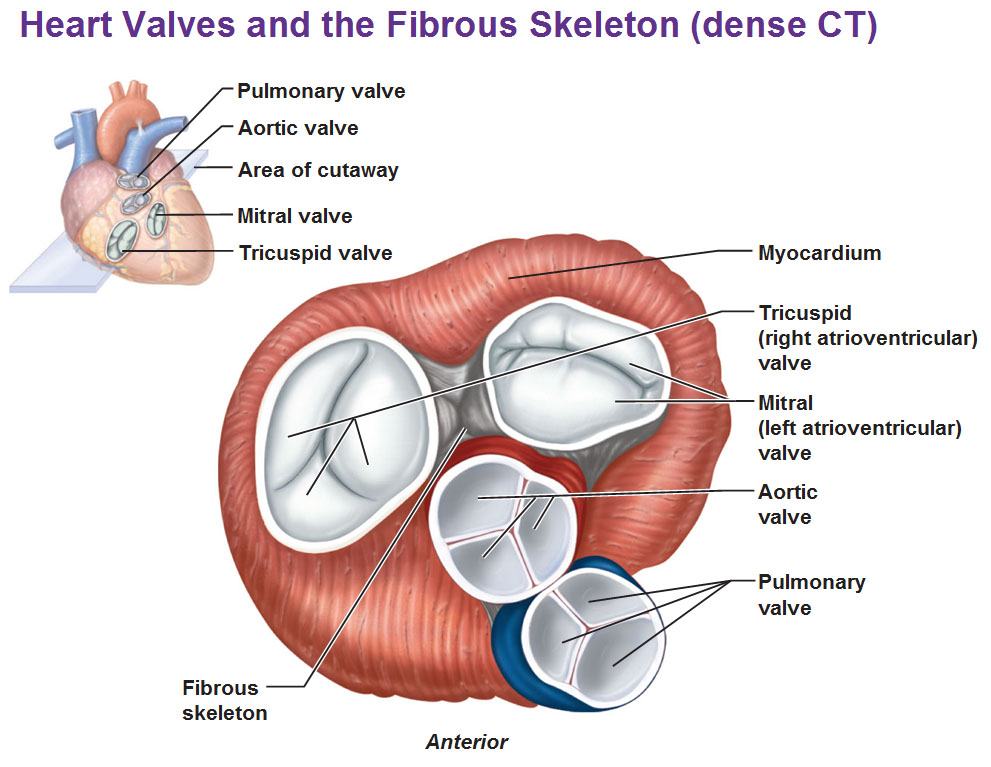
- Valves (🚪 ensure one-way flow):
- AV Valves: Tricuspid (RA→RV), Mitral (LA→LV).
- Semilunar Valves: Pulmonary (RV→PA), Aortic (LV→Aorta).
Heart Borders & Surfaces 📐
- Right border = Right atrium
- Left border = Left ventricle (plus LA appendage)
- Inferior surface = Both ventricles resting on diaphragm
- Base (posterior) = Left atrium (important landmark in TOE)
📌 Exam Tip: Dysphagia + hoarseness in LA enlargement → compression of oesophagus & left recurrent laryngeal nerve (Ortner’s syndrome).
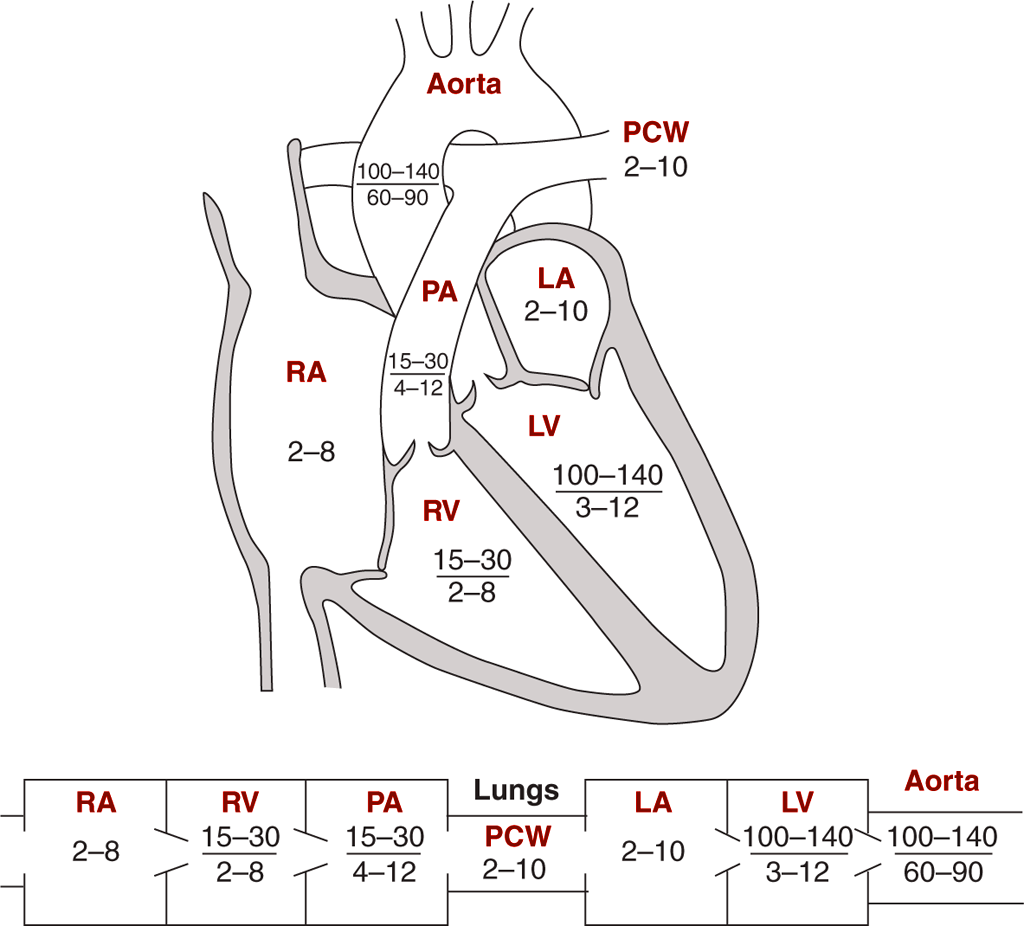
The Cardiac Cycle 🔄
- Systole: Isovolumetric contraction → ventricular ejection.
- Diastole: Isovolumetric relaxation → passive filling → atrial systole.
📌 Exam Tip: Most coronary perfusion occurs during diastole – hence why tachycardia can precipitate angina.
Electrical Conduction ⚡
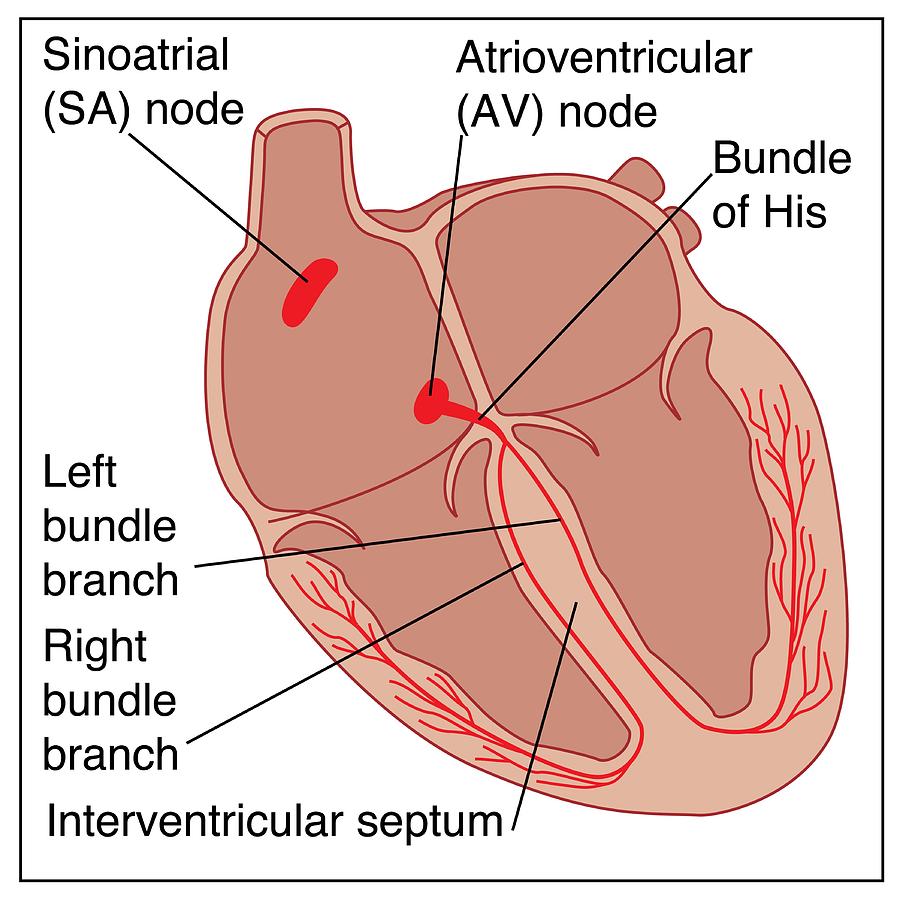
- SA node (pacemaker, 60–100 bpm)
- AV node (delays impulse for ventricular filling)
- Bundle of His → Bundle branches → Purkinje fibres
📌 Exam Tip: Complete heart block = failure of AV conduction → ventricular escape rhythm (20–40 bpm).
Action Potentials 📊
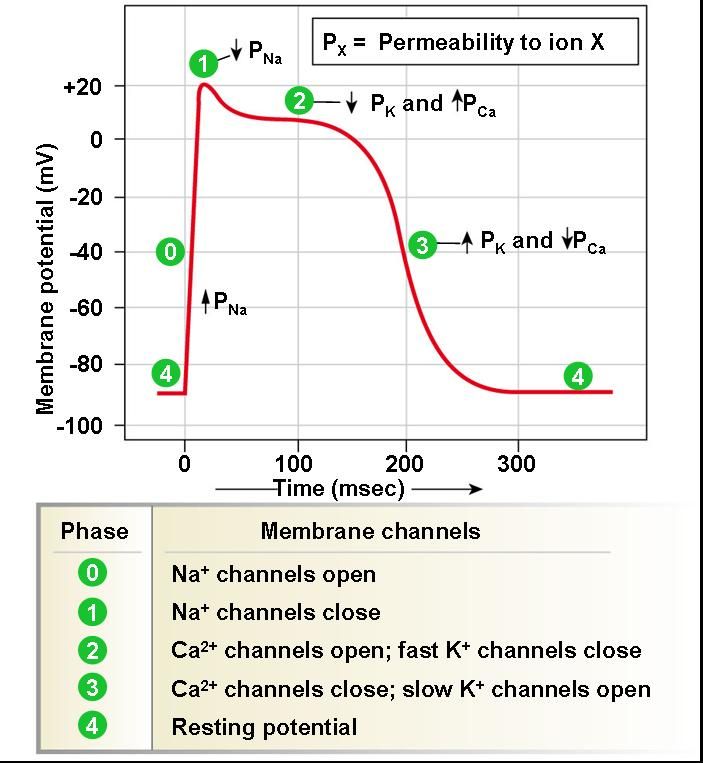
- Pacemaker cells: spontaneous depolarisation (Na⁺ & Ca²⁺ in), repolarisation (K⁺ out).
- Ventricular myocytes: fast Na⁺ depolarisation, plateau (Ca²⁺ in vs K⁺ out), repolarisation.
📌 Exam Tip: Plateau phase (Ca²⁺ influx) explains why tetany doesn’t occur in cardiac muscle.
Frank–Starling Law 📈
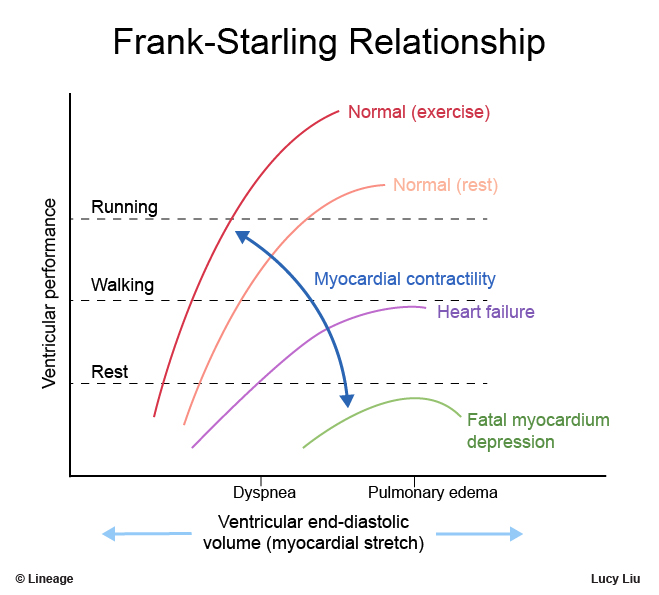
Within limits, ↑ preload = ↑ stroke volume. Curve flattens in heart failure → pulmonary oedema risk.
Coronary Circulation 🩸
- LAD → anterior LV & septum.
- Circumflex → lateral LV.
- RCA → RV, inferior LV (in right-dominant hearts, 70%).
📌 Exam Tip: RCA occlusion → inferior MI → may cause AV block (as AV node is RCA supplied in most patients).
Autonomic & Hormonal Regulation 🧠
- Sympathetic → ↑ HR, contractility (β₁).
- Parasympathetic (vagus) → ↓ HR, conduction.
- RAAS → vasoconstriction & fluid retention.
- BNP/ANP → vasodilation & natriuresis (useful biomarkers in HF).
Exercise Physiology 🏃
- ↑ HR & ↑ SV → ↑ CO up to 20–25 L/min.
- Redistribution of blood to muscle & skin.
Clinical Correlations 🩺
- Heart failure → reduced CO, neurohormonal activation.
- Arrhythmias → compromise cardiac output (e.g., AF → loss of atrial systole).
- Ischaemic heart disease → angina, MI, arrhythmias.
- Valvular disease → murmurs & altered hemodynamics.
📌 Exam Tip: S3 = volume overload (HF), S4 = stiff ventricle (HTN, aortic stenosis).