Makindo Medical Notes"One small step for man, one large step for Makindo" |
|
|---|---|
| Download all this content in the Apps now Android App and Apple iPhone/Pad App | |
| MEDICAL DISCLAIMER: The contents are under continuing development and improvements and despite all efforts may contain errors of omission or fact. This is not to be used for the assessment, diagnosis, or management of patients. It should not be regarded as medical advice by healthcare workers or laypeople. It is for educational purposes only. Please adhere to your local protocols. Use the BNF for drug information. If you are unwell please seek urgent healthcare advice. If you do not accept this then please do not use the website. Makindo Ltd. |
Hydrogen and other Bonds
-
| About | Anaesthetics and Critical Care | Anatomy | Biochemistry | Cardiology | Clinical Cases | CompSci | Crib | Dermatology | Differentials | Drugs | ENT | Electrocardiogram | Embryology | Emergency Medicine | Endocrinology | Ethics | Foundation Doctors | Gastroenterology | General Information | General Practice | Genetics | Geriatric Medicine | Guidelines | Haematology | Hepatology | Immunology | Infectious Diseases | Infographic | Investigations | Lists | Microbiology | Miscellaneous | Nephrology | Neuroanatomy | Neurology | Nutrition | OSCE | Obstetrics Gynaecology | Oncology | Ophthalmology | Oral Medicine and Dentistry | Paediatrics | Palliative | Pathology | Pharmacology | Physiology | Procedures | Psychiatry | Radiology | Respiratory | Resuscitation | Rheumatology | Statistics and Research | Stroke | Surgery | Toxicology | Trauma and Orthopaedics | Twitter | Urology
Related Subjects: |Carbohydrates |Hydrogen and other Bonds
🌍 Life depends on chemistry: every heartbeat, thought, and breath reflects countless atoms of oxygen, carbon, hydrogen, and other elements forming and breaking bonds. ⚡ These dynamic interactions create the structure of biomolecules and drive metabolism, energy transfer, and signalling.
🔗 Types of Chemical Bonds
- ⚡ Ionic Bonds:
Ions are charged atoms formed when one atom donates or accepts electrons.
- Positive = cation (e.g., Na⁺)
- Negative = anion (e.g., Cl⁻)
- Opposite charges attract → form ionic bonds (e.g., NaCl crystals).
- Biological relevance: Key in nerve conduction, muscle contraction, and electrolyte balance.
- 🔗 Covalent Bonds:
Electrons are shared between atoms.
- Nonpolar covalent bonds: Equal sharing of electrons → electrically neutral molecules (e.g., O₂, CH₄). 🧪 Basis of lipid membranes (hydrophobic tails).
- Polar covalent bonds: Unequal sharing → “poles” of charge (e.g., H₂O). 💧 This underlies solubility and hydrogen bonding.
- Biological relevance: Stable backbone of DNA, proteins, carbohydrates.
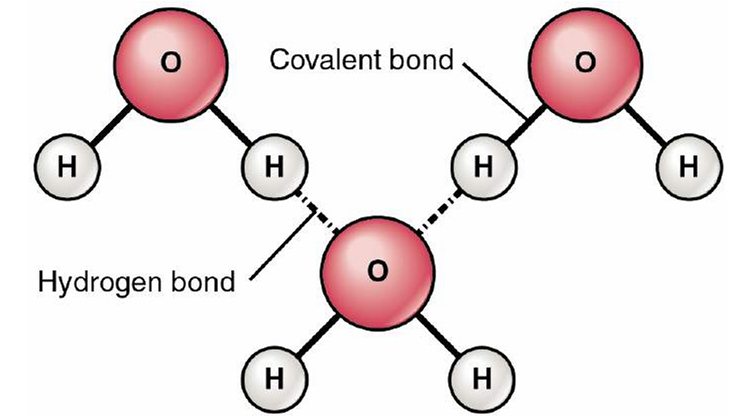
- 💧 Hydrogen Bonds:
A weak but vital interaction when a hydrogen atom covalently bound to an electronegative atom (O, N) is attracted to another electronegative atom.
- Weaker than covalent/ionic bonds but crucial for life.
- Stabilises DNA double helix, protein secondary structures (α-helix, β-sheet).
- Gives water its unique properties: high boiling point, surface tension, excellent solvent.
🧬 Integration in Biology
In living organisms, bonds constantly form and break to allow flexibility and energy flow:
- 💧 Water: Polar covalent bonds → hydrogen bonding → solvent of life.
- 🧂 Ionic interactions: Salt (NaCl) dissolves via ion-dipole interactions with water.
- 🥑 Nonpolar covalent bonds: Hydrophobic lipids cluster in membranes → essential for cell structure.
- 🧩 Proteins: Hydrogen bonds + ionic interactions stabilise folding and enzyme function.
📊 Clinical & Physiological Relevance
- ⚡ Electrolyte imbalance: Low Na⁺, K⁺, or Ca²⁺ disrupt ionic gradients → arrhythmias, seizures.
- 🧬 DNA damage: Breaking hydrogen bonds → mutation or apoptosis.
- 🌡️ Fever: Alters hydrogen bonding in proteins → denaturation at high temperatures.
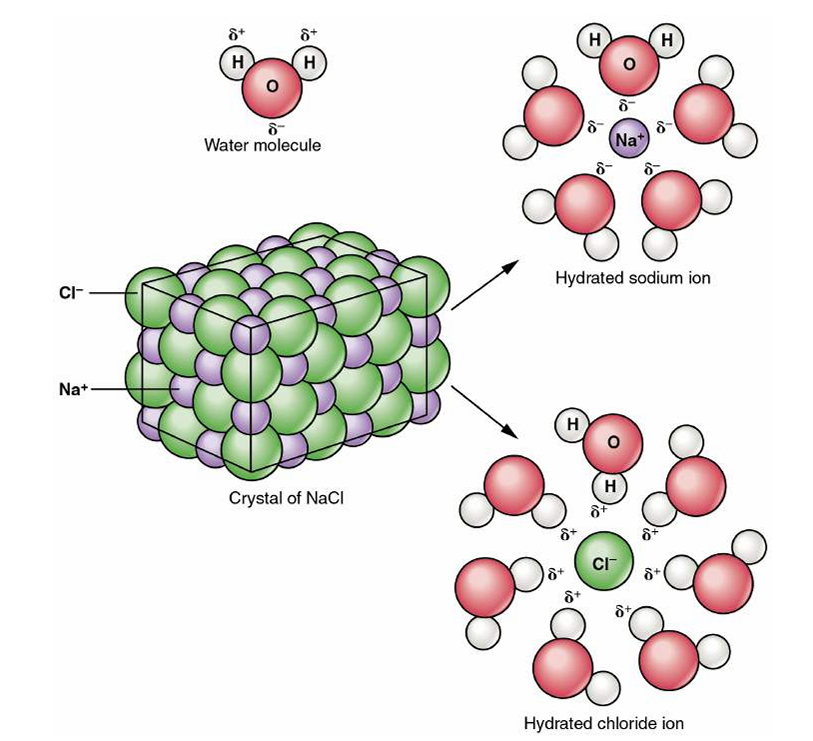
🧂 Dissociation of Sodium Chloride in Water
NaCl does not dissolve as intact molecules but dissociates into Na⁺ cations and Cl⁻ anions, each surrounded by water molecules (hydration shells) → stabilising ions in solution. 🔑 This principle explains conduction in nerves and osmotic gradients.
✅ Conclusion
From ionic Na⁺/Cl⁻ gradients powering nerve impulses, to hydrogen bonds shaping DNA and proteins, chemical bonds are the language of biology. 🔑 Understanding these interactions helps explain everything from cell signalling and drug action to genetic inheritance and disease.