Makindo Medical Notes"One small step for man, one large step for Makindo" |
|
|---|---|
| Download all this content in the Apps now Android App and Apple iPhone/Pad App | |
| MEDICAL DISCLAIMER: The contents are under continuing development and improvements and despite all efforts may contain errors of omission or fact. This is not to be used for the assessment, diagnosis, or management of patients. It should not be regarded as medical advice by healthcare workers or laypeople. It is for educational purposes only. Please adhere to your local protocols. Use the BNF for drug information. If you are unwell please seek urgent healthcare advice. If you do not accept this then please do not use the website. Makindo Ltd. |
Caspases
-
| About | Anaesthetics and Critical Care | Anatomy | Biochemistry | Cardiology | Clinical Cases | CompSci | Crib | Dermatology | Differentials | Drugs | ENT | Electrocardiogram | Embryology | Emergency Medicine | Endocrinology | Ethics | Foundation Doctors | Gastroenterology | General Information | General Practice | Genetics | Geriatric Medicine | Guidelines | Haematology | Hepatology | Immunology | Infectious Diseases | Infographic | Investigations | Lists | Microbiology | Miscellaneous | Nephrology | Neuroanatomy | Neurology | Nutrition | OSCE | Obstetrics Gynaecology | Oncology | Ophthalmology | Oral Medicine and Dentistry | Paediatrics | Palliative | Pathology | Pharmacology | Physiology | Procedures | Psychiatry | Radiology | Respiratory | Resuscitation | Rheumatology | Statistics and Research | Stroke | Surgery | Toxicology | Trauma and Orthopaedics | Twitter | Urology
Caspases are a family of protease enzymes that play essential roles in programmed cell death (apoptosis) and inflammation. They are involved in the initiation and execution of apoptosis, as well as in the processing of pro-inflammatory cytokines.
Structure of Caspases
- Pro-caspases :
- Caspases are initially synthesized as inactive pro-caspases.
- Pro-caspases contain an N-terminal prodomain, a large subunit, and a small subunit.
- Activation :
- Activation involves cleavage of the pro-caspase at specific aspartate residues.
- The cleavage releases the prodomain and allows the large and small subunits to form an active enzyme complex.
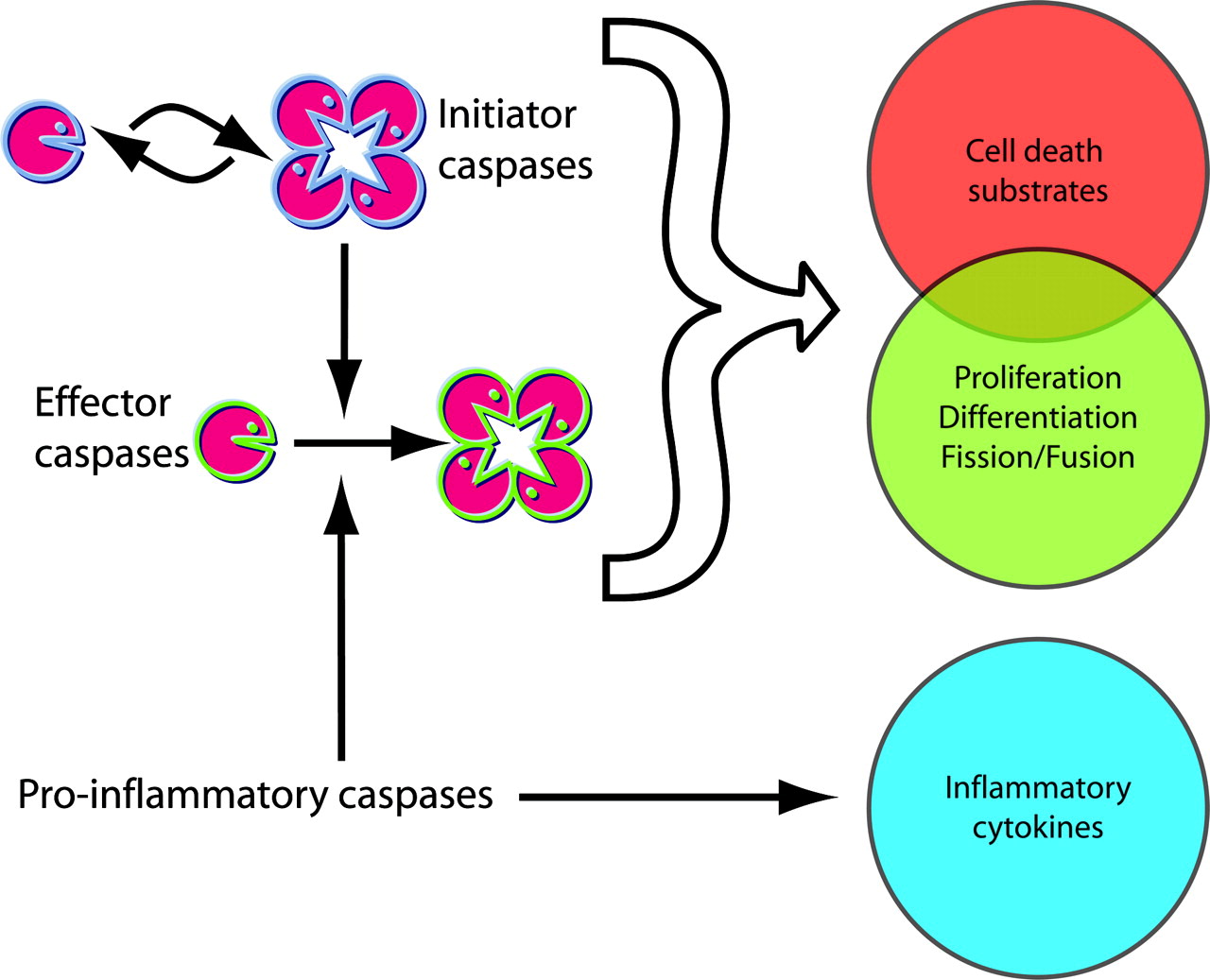
Classification of Caspases
- Initiator Caspases :
- Include caspases-2, -8, -9, and -10.
- Initiate the apoptotic process by responding to apoptotic signals and activating effector caspases.
- Effector Caspases :
- Include caspases-3, -6, and -7.
- Execute apoptosis by cleaving various cellular substrates leading to cell death.
- Inflammatory Caspases :
- Include caspases-1, -4, -5, and -12.
- Involved in the processing of pro-inflammatory cytokines such as interleukin-1β (IL-1β) and interleukin-18 (IL-18).
Pathways of Apoptosis
- Intrinsic Pathway (Mitochondrial Pathway) :
- Triggered by internal stress signals such as DNA damage, oxidative stress, or developmental cues.
- Involves the release of cytochrome c from mitochondria, which forms the apoptosome with Apaf-1 and pro-caspase-9, leading to the activation of caspase-9.
- Extrinsic Pathway (Death Receptor Pathway) :
- Triggered by external signals such as binding of death ligands (e.g., FasL, TNF-α) to death receptors (e.g., Fas, TNFR).
- Leads to the formation of the death-inducing signaling complex (DISC) and the activation of caspase-8.
Caspase Activation
- Cleavage :
- Caspases are activated by proteolytic cleavage at specific aspartate residues.
- Cleavage separates the large and small subunits, which then dimerize to form the active enzyme.
- Oligomerization :
- Initiator caspases form oligomeric complexes (e.g., apoptosome, DISC) that facilitate their activation.
Functions of Caspases
- Execution of Apoptosis :
- Effector caspases cleave various cellular substrates, leading to the characteristic morphological and biochemical features of apoptosis.
- Cleavage targets include nuclear proteins, cytoskeletal proteins, and DNA repair enzymes.
- Inflammatory Response :
- Inflammatory caspases process pro-inflammatory cytokines such as IL-1β and IL-18 into their active forms.
- Play a role in the immune response and inflammation.
Regulation of Caspase Activity
- Inhibitors of Apoptosis Proteins (IAPs) :
- Bind to and inhibit active caspases to prevent apoptosis.
- Anti-apoptotic Bcl-2 Family Proteins :
- Regulate the intrinsic pathway by preventing the release of cytochrome c from mitochondria.
- Pro-apoptotic Bcl-2 Family Proteins :
- Promote the intrinsic pathway by facilitating the release of cytochrome c from mitochondria.
Clinical Significance
- Cancer :
- Deregulation of caspase activity can lead to the survival of abnormal cells and contribute to cancer development.
- Caspases are being explored as therapeutic targets for cancer treatment.
- Neurodegenerative Diseases :
- Excessive activation of caspases can contribute to the death of neurons in diseases such as Alzheimer's, Parkinson's, and Huntington's diseases.
- Autoimmune Diseases :
- Abnormal regulation of apoptosis can lead to the survival of autoreactive immune cells, contributing to autoimmune diseases.
Summary
Caspases are crucial enzymes involved in the processes of apoptosis and inflammation. They are synthesized as inactive pro-caspases and activated by proteolytic cleavage. Caspases are classified into initiator, effector, and inflammatory caspases based on their roles. They function in executing apoptosis, processing pro-inflammatory cytokines, and regulating immune responses. Dysregulation of caspase activity is implicated in various diseases, including cancer, neurodegenerative disorders, and autoimmune diseases, making them important targets for therapeutic interventions.