Makindo Medical Notes"One small step for man, one large step for Makindo" |
|
|---|---|
| Download all this content in the Apps now Android App and Apple iPhone/Pad App | |
| MEDICAL DISCLAIMER: The contents are under continuing development and improvements and despite all efforts may contain errors of omission or fact. This is not to be used for the assessment, diagnosis, or management of patients. It should not be regarded as medical advice by healthcare workers or laypeople. It is for educational purposes only. Please adhere to your local protocols. Use the BNF for drug information. If you are unwell please seek urgent healthcare advice. If you do not accept this then please do not use the website. Makindo Ltd. |
Immunoglobulins and their role in Immunity
-
| About | Anaesthetics and Critical Care | Anatomy | Biochemistry | Cardiology | Clinical Cases | CompSci | Crib | Dermatology | Differentials | Drugs | ENT | Electrocardiogram | Embryology | Emergency Medicine | Endocrinology | Ethics | Foundation Doctors | Gastroenterology | General Information | General Practice | Genetics | Geriatric Medicine | Guidelines | Haematology | Hepatology | Immunology | Infectious Diseases | Infographic | Investigations | Lists | Microbiology | Miscellaneous | Nephrology | Neuroanatomy | Neurology | Nutrition | OSCE | Obstetrics Gynaecology | Oncology | Ophthalmology | Oral Medicine and Dentistry | Paediatrics | Palliative | Pathology | Pharmacology | Physiology | Procedures | Psychiatry | Radiology | Respiratory | Resuscitation | Rheumatology | Statistics and Research | Stroke | Surgery | Toxicology | Trauma and Orthopaedics | Twitter | Urology
Immunoglobulins (Ig), also known as antibodies, are glycoprotein molecules produced by plasma cells. They play a crucial role in the immune system by recognizing and binding to specific antigens, such as pathogens or foreign substances, and facilitating their neutralization or destruction. Two identical heavy and light (H-L) chain combinations are also held together by disulfide bridges forming a basic four-chain (H-L)2 antibody structure, a dimer of dimers. Basically, an antibody molecule has two functions i.e., antigen binding and effector functions. The binding of an antibody with an antigen is very specific (i.e., a single antibody can not bind with different antigens/epitopes) which is determined by the structural configuration of the antigen-binding region of that antibody.
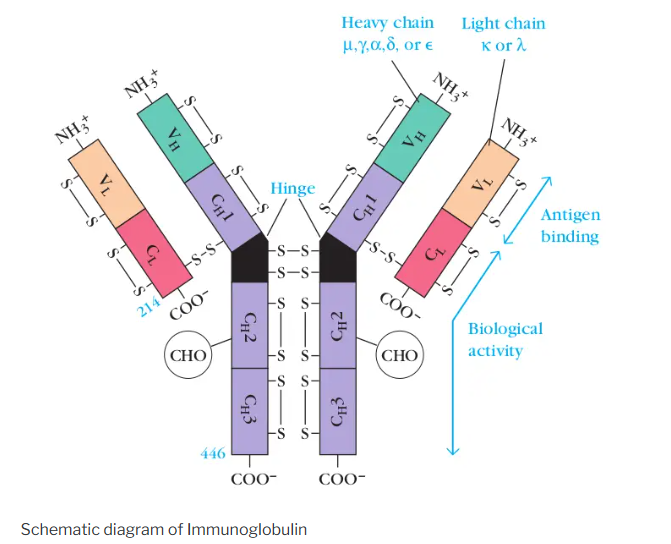
Basic Structure of Immunoglobulins
- Heavy and Light Chains :
- Immunoglobulins consist of two heavy chains and two light chains.
- The heavy chains are identical to each other, and the light chains are identical to each other.
- Light chains are called light chains because their molecular weight is less i.e. about 25,000.
- Molecular weight of heavy chains is 50,000 to 70,000 depending upon antibody isotype/class.
- Variable (V) and Constant (C) Regions :
- Each chain has a variable region (V) and a constant region (C).
- The variable region determines the antigen specificity of the antibody.
- The constant region mediates effector functions by interacting with other components of the immune system.
- Fab and Fc Fragments :
- The antibody can be enzymatically cleaved into two Fab fragments and one Fc fragment.
- Fab (Fragment antigen-binding) contains the variable regions and binds to antigens.
- Fc (Fragment crystallizable) contains the constant regions and mediates effector functions.
- Hinge Region :
- A flexible region between the Fab and Fc fragments that allows the antibody to adopt different conformations.
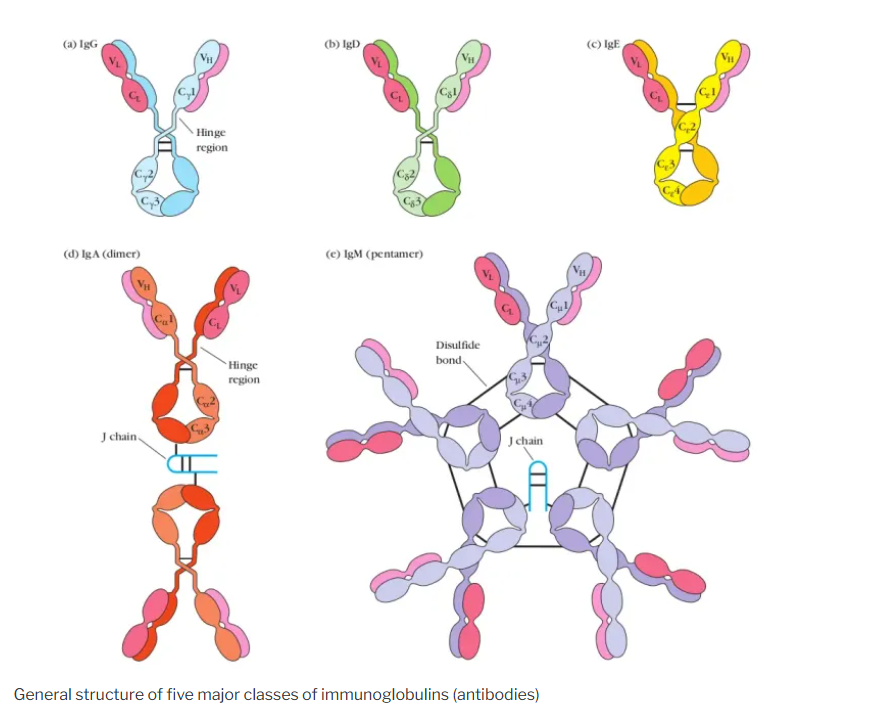
Classes and Subclasses of Immunoglobulins
- IgG :
- The most abundant class of immunoglobulins in the blood and extracellular fluid.
- Has four subclasses: IgG1, IgG2, IgG3, and IgG4.
- Functions: Opsonization, neutralization, antibody-dependent cellular cytotoxicity (ADCC), and complement activation.
- IgA :
- Found in mucosal areas, such as the gut, respiratory tract, and urogenital tract, as well as in saliva, tears, and breast milk.
- Exists in monomeric and dimeric forms.
- Functions: Mucosal immunity and neutralization of pathogens.
- IgM :
- The first antibody produced in response to an infection.
- Exists as a pentamer, with five monomer units joined together.
- Functions: Primary immune response and complement activation.
- IgE :
- Involved in allergic reactions and defense against parasitic infections.
- Functions: Binding to allergens and triggering histamine release from mast cells and basophils.
- IgD :
- Found in small amounts in the blood and on the surface of B cells.
- Functions: B cell receptor (BCR) involved in B cell activation.
Genetic Basis of Immunoglobulin Variation
- V(D)J Recombination :
- Process by which B cells generate diverse antibody repertoires.
- Involves the random recombination of variable (V), diversity (D), and joining (J) gene segments for heavy chains and V and J segments for light chains.
- Somatic Hypermutation :
- Occurs in activated B cells during an immune response.
- Introduces point mutations in the variable regions of immunoglobulin genes, leading to increased antibody affinity for the antigen.
- Class Switch Recombination :
- Process by which B cells change the class of antibody they produce without altering the antigen specificity.
- Involves recombination of the constant region gene segments.
Functions of Immunoglobulins
- Neutralization :
- Antibodies bind to pathogens or toxins, preventing them from interacting with host cells.
- Opsonization :
- Antibodies coat pathogens, enhancing their recognition and ingestion by phagocytes.
- Complement Activation :
- Antibodies activate the complement system, leading to pathogen lysis and inflammation.
- Antibody-Dependent Cellular Cytotoxicity (ADCC) :
- Antibodies bind to infected or cancerous cells, recruiting natural killer (NK) cells to destroy them.
- Agglutination and Precipitation :
- Antibodies cross-link antigens, forming large complexes that are easier to clear from the body.
Clinical Applications
- Monoclonal Antibodies :
- Antibodies produced from a single clone of B cells, used in the treatment of cancers, autoimmune diseases, and infectious diseases.
- Immunoglobulin Therapy :
- Administration of pooled immunoglobulins from healthy donors to treat immunodeficiency disorders and certain autoimmune diseases.
- Diagnostic Tests :
- Detection of specific antibodies in the blood can help diagnose infections, autoimmune diseases, and allergies.
Summary
Immunoglobulins are essential components of the immune system, with a basic structure comprising two heavy and two light chains, each with variable and constant regions. There are five main classes of immunoglobulins: IgG, IgA, IgM, IgE, and IgD, each with distinct functions and locations in the body. Genetic mechanisms such as V(D)J recombination, somatic hypermutation, and class switch recombination generate antibody diversity. Immunoglobulins play crucial roles in neutralization, opsonization, complement activation, ADCC, and agglutination. Clinically, they are used in therapies, diagnostics, and treatments for various diseases.